by
Jón Kristjánsson
Fiski- Fisheries Management, Reykjavík Iceland.
E-mail: jon@fiski.com - URL: http://www.fiski.com
January 2001
The shrimp fishery at Flemish Cap started in 1993 when 28.000 tons were caught. From 1994- 1998 the yearly average was some 32.000 tons with a peak of 48.000 tons in 1996. The catch in 1999 was 42.000 tons and increased to around 50.000 tons in 2000 (Anon 2000) . Skuladottir et. al. (1999 a) gave an overview of the international fishery for shrimp on Flemish Cap.
In November 2000, the Scientific Council advised that the catch should be reduced to 30.000 tons in 2001, partly because they considered the 97- year class to be below average (Anon. 2000).
The advices of the Scientific Council will be viewed in the light of the observed changes in stock parameters from 1995 to 2000.
Samples were collected by the author on board Icelandic and Estonian shrimp trawlers during commercial fishing in the years 1995, 1996, 1999 and 2000. The samples were taken unselected from the bunker. The 1997 samples were collected by crew member, same procedure used, and sent frozen to Iceland for analysis in the laboratory. The oblique carapace length of shrimp (OCL) was measured to the nearest 0.1 mm, sorted into 0.5 mm length classes and separated into sex categories, i.e. males, transitionals, and females.
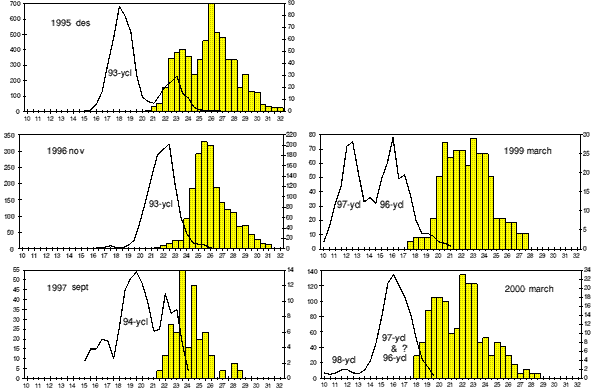
Since 1995, changes in vital stock parameters are reflected in samples from the commercial catch (Figure 1). These are growth rate of males, size at sex change and maximum length of females .
Mean length of 2 year old males in November 1995 was 18.6 mm. A year later the same year class, then age 3, was 22.0 mm. This gives the growth rate G length = 0.17 which corresponds to G weight = 0.5.
In March 1999 the mean length of the 96- year class (3 years old, all males) was around 16 mm, the 97- year class (2+) was 12.5 mm. This means that 3+ shrimp in late autumn 1999 are smaller than 2+ shrimp were at the same time of the year in 1995.
Samples from March 2000 do not show any distinct length separation of the 97- and 96- cohorts. It is possible that at least a part of the 96- year class is clustered together with the 97- year class in the length interval 15-18 mm OCL. If so, then a possible explanation is declining growth of the 96- year class.

Figure 1. Relative length distribution of male and intermediate shrimp combined (line) and female shrimp (bars) in the catch at Flemish Cap in 1995-2000. Note that males and females are different scale, no. males on vertical axis to the left and no. females on the axis to the right in the figures. The x-axis is OCL in mm.
Growth of the 94- year class in the first years of its life was slower than the growth of the 93- year class was in the same period of its life. This is shown in figure 2 a) which shows the mean length of these year classes lengths at different times.
Part of the 93- year class changed sex in the winter 1996-97. In figure 2 b) it can be seen that shrimp becoming females (transitionals) are larger than those continuing as males.
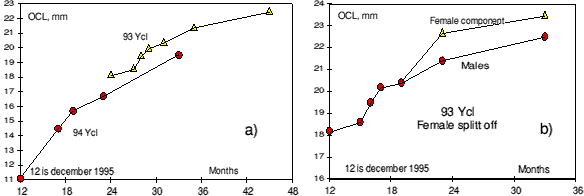
Figure 2. a) Growth of the 93- and 94- year classes. Size is plotted against age in months. The 94- year class is considered to be 12 months old in December 1995. All 94- shrimp were males, part of the 93- shrimp were changing sex at age of 36 months. b) Shows how the 93- year class splits into two categories: Fast growing part, intermediates becoming females and slow growing part, remaining males.
The separation of the fast growing part of the of the 93- year class to become transitionals is shown in more detail in figure 3. This finding supports that the size rather than age determines the onset of sex change (female maturity)
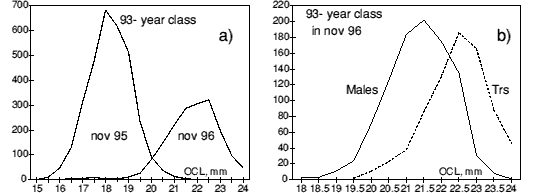
Figure 3 a) Length distribution of the 93- year class in November 1995, then all males, and in November 1996, males and transitionals combined. b) Length distribution of the 93- year class split into males (unbroken line) and transitionals (Trs).
Size of the smallest egg bearing females has decreased from 20.5 mm OCL in November 1995 to 18.0 mm in March 2000 (Figure 1). The maximum size of shrimp has also decreased from 32 mm OCL in 1995 to 28.5 mm in 2000. The ratio minimum/ maximum size of females is however unchanged, 0.64 in 1995 and 0.63 in 2000.
The samples are taken at different locations different years and as size of shrimp is known to vary by depth, the samples may not be exactly comparable. The data however show a definite trend in life history parameters.
Slower growth, reduced size at sex change and smaller maximum size of shrimp all point in the same direction: Growth conditions for the individual shrimp have worsened since 1995.
Retarded growth from 1993-1999 is supported by Skuladottir et. al. (1999) who calculated the length at age for shrimp at Flemish Cap 1993 - 1999. Figure 4. is a plot of weight at ages 1-5 1993-1995 as presented in table 3 (Skuladottir et. al. 1999).
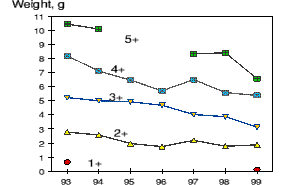
Figure 4. Weight at age of shrimp from table 3 in Skuladottir et. al. (1999) .
Environmental conditions, such as temperature, availability of food and competition determine the the growth rate.
Size of sex change in shrimp can be considered to be parallel to size at maturity in fish, because in shrimp, the egg and embryo production is the energy consuming part of the reproduction process. Onset of maturity in fish is related to growth and it is generally accepted that growth of individual fish decreases at or after the onset of maturity for then often to cease altogether. This calls for an explanation.
Size at sexual maturity is a highly variable feature within a species. It may vary considerably from one stock to another, and it may also be variable with time, reflecting temporal changes in living conditions.
There is not one generally accepted view on how growth relates to maturity. In some cases it appears that the fish that grow fastest in early life mature earliest, and may mature at a smaller size than those who grow more slowly. This is usually most obvious in a pond where all the fish in the pond are of the same year class. In a given environment there are two basic things that limit the growth of a fish, i.e. availability and access to food and the size of the fish. In a uniform habitat (e.g. a pond), there is little opportunity for niche shifts. At a certain size, the environment cannot support more growth and this brings on the onset of maturity. In general then one can say that reduced growth brings on maturity causing further reduction in growth rate. Rate of growth thus generally decreases with increased size, a process that can usually be adequately described by the von Bertalanffy growth equation.
The connection between growth and maturity has been extensively studied by several authors, and the relationship between size at sexual maturity and other population parameters is well known (Holt 1962, Beverton 1992). It is widely assumed that maturity brings on reduced growth, but several authors have challenged that view. They consider maturity to be the inevitable consequence of reduced potential for growth that follows increased body size if there is no ontogenetic niche shift (Alm 1959, Iles 1974, Pauly 1981).
The slower growers sometimes mature at a larger size than those growing faster because in many species, the breeding season is well defined so the decision on whether to mature or not is only taken once a year. In a more heterogenous environment there are more available niches for fish than in the pond or limited environment. How the fish can use these niches depends on their adaptations (species) and within a species, on their size (Parker and Larkin 1959).
There is an inverse relationship between body size and mortality in marine fish (Peterson and Wroblewski 1984). Fishing of virgin populations often leads to increased recruitment, which in turn leads to reduced growth and reduced size at sexual maturity or stunting, especially when the largest individuals of the population are selectively removed (Langeland and Jonsson 1988).
The Flemish Cap can hardly be described as a pond but the area suitable for shrimp is limited and growth rate can become density dependant. If retarded growth in shrimp at Flemish Cap cannot be explained by change in environmental conditions, selective fishing or underfishing could be the cause. Present continued fishing pattern may eventually change the size composition of the stock to a further degree, i.e. maximum size may be further reduced and the average size of shrimp become lower.
Following hypothesis can be put forward:
When larger individuals are removed from a fish stock by selective fishing competition (and cannibalism) decreases which in turn leads to increased recruitment. This leads to changes in stock composition towards smaller individual size and retarded growth.
This situation is often misinterpreted as result of overfishing. A remedy to correct such a "skewed" fish stock would be to use unselective gear or combination of fishing methods that lead to unselective removals from the stock (Kolding 1994).
Therefore, a management directed towards increased fishing pressure on small shrimp should be considered, as reduced fishing pressure on larger shrimp may not be practical or even possible. This is contrary to the existing management strategies, which suggest closure of areas with small shrimp and regards large year classes as a positive sign for the development of the stock.
Alm, G. 1959. Connection between maturity, size and age in fishes. Institute of Freshwater Research, Drottningholm. Report 40: 5-145.
Anon. 2000. Report of the Scientific Council Meeting November 2000 SCD Doc. 00/27, Serial No. N4345
Beverton, R.J.H. 1992. Patterns of reproductive strategy parameters in some marine teleost fishes. Journal of Fish Biology (1992) 41 (Supplement B), 137-160.
Holt, S.J. 1962. The application of comparative population studies to fisheries biology - an exploration. In Le Cren E.D. and M.W. Holdgate (eds.). The exploitation of natural animal populations. Blackwell Scientific Publications, Oxford. pp. 51-71.
Iles, T.D. 1974. The tactics and strategy of growth in fishes. In Harden Jones, F.R. (ed.). Sea Fisheries Research. John Wiley and Sons, N.Y. pp. 331-345.
Kolding, J. 1994. Plus ca change, plus c'est la meme chose. On the ecology and exploitation of fish in fluctuating tropical freshwater systems. Unpublished Ph.D. thesis, University of Bergen, Norway.
Langeland, A. & Jonsson, 1988. Management of stunted populations of Arctic charr (Salvelinus alpinus) and brown trout ( Salmo trutta) in Norway. In: W.L.T. van Densen, B. Steinsmertz & R.H. Huges (Eds). Management of freshwater fisheries. Proceedings of a symposium organized by the European Inland Fisheries Advisory Commission, Göteborg, Sweden, 31 May-3 June 1988. Pudoc. Wageningen. pp 396-405.
Parker, R.R. and P.A. Larkin, 1959. A concept of growth in fishes. Journal of the Fisheries Research Board of Canada. 16: 333-355.
Pauly, D. 1981. The relationships between gill surface area and growth performance in fish: a generalization of von Bertalanffy's theory of growth. Meeresforch. 28 (1981) 251-282.
Peterson, I. and J.S. Wroblewski, 1984. Mortality rate of fishes in the pelagic ecosystem. Canadian Journal of Fisheries and Aquatic Sciences 41: 1117-1120.
Skuladottir U., D.G. Parsons and D. Orr 1999. The International Fishery for Shrimp (Pandalus borealis) in Division 3M (Flemish Cap), 1993-1999. NAFO SCR Doc. 9/112 Serial No. N4192. 21p.